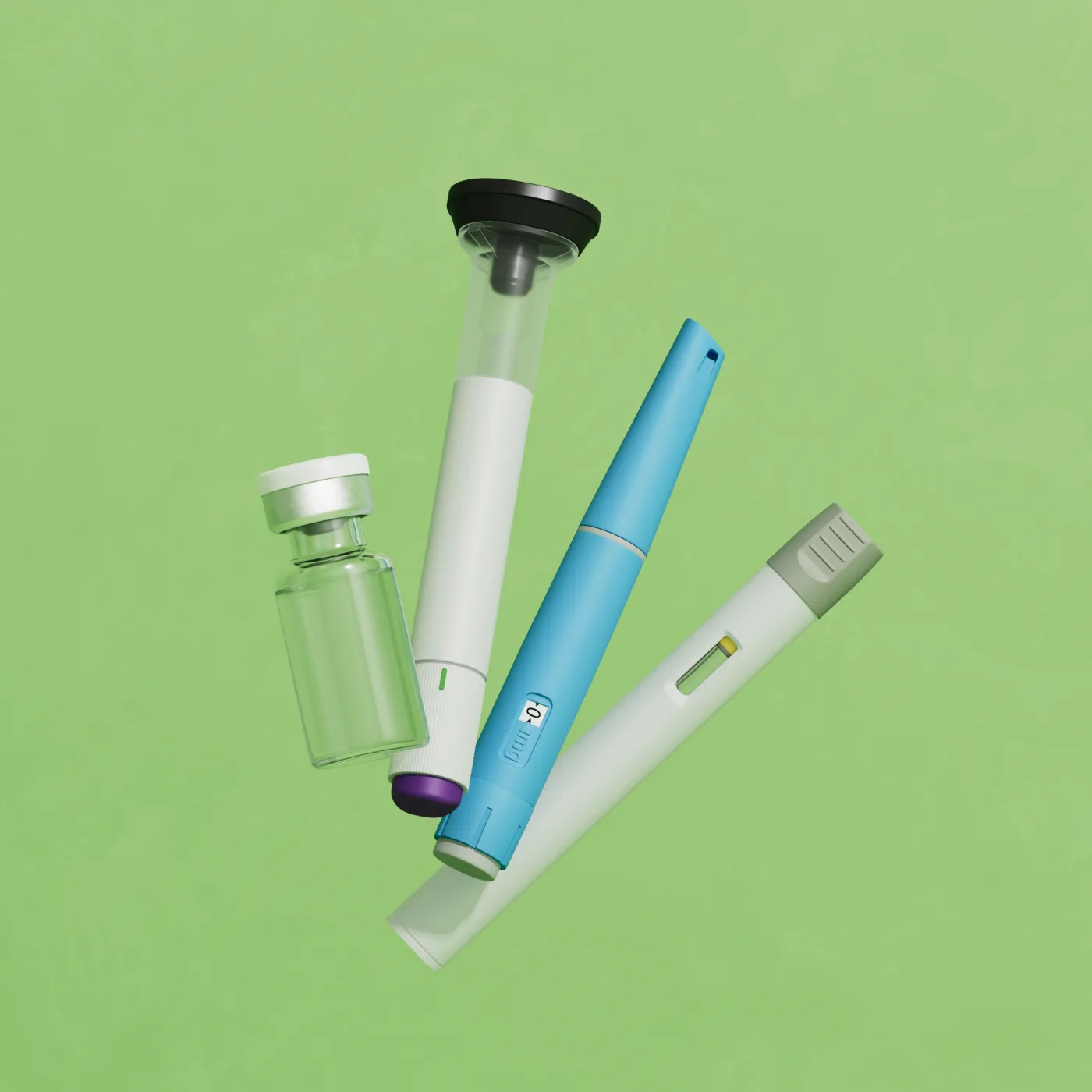
GLP-1 Treatments
Personalized GLP‑1 Treatments
Your goals, your plan
A personalized weight loss plan built around you
GLP-1 medications may be preferred over other weight loss drugs due to their non-stimulant nature, reducing the risk of side effects related to stimulant medications. Unlike stimulants such as phentermine, GLP-1s may aid in weight loss while also improving blood sugar control. The suitability of medications varies depending on your health profile. Start your free telehealth consultation to discuss available medication options with a licensed provider.
Every Eden plan is built around you. You’ll receive a personalized treatment plan created by a licensed medical provider after a full review of your health profile. If medication is right for you, we’ll craft a custom dosing schedule and ship your treatment straight to your door, through a certified, state-licensed pharmacy. You’ll also get unlimited 24/7 messaging with your dedicated care team - so you’re supported every step of the way.
There are several FDA-approved prescription medications that may be considered by licensed providers for individuals with obesity, a high BMI, or weight-related health conditions. These may include medications with active ingredients such as semaglutide, tirzepatide, liraglutide, setmelanotide, phentermine, orlistat, and naltrexone-bupropion. In certain cases, other medications, including some antidepressants, may be prescribed off-label. All treatment decisions are made by licensed providers based on clinical evaluation and individual health needs.
Your Eden-licensed medical provider will evaluate your health profile and determine whether a prescription medication may be an appropriate part of your overall treatment plan. Some programs offer access to branded GLP-1 medications that are injectable, as well as personalized oral medication kits that may include ingredients like metformin, bupropion, topiramate, vitamin B12, or naltrexone. These oral medications are typically taken once daily and may work in different ways to support weight management.
You can easily cancel your treatment plan from within your online patient portal. There are no cancellation fees or long-term contracts. Please note that canceling your treatment plan in the portal will not cancel any active orders that have already been sent to the pharmacy. If your order is in a pending status, please contact us directly at [email protected] for assistance.
The statements on this page have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
**The FDA does not review or approve any compounded medications for safety or effectiveness.
Medication made affordable
Eden connects patients with licensed providers who may prescribe medication through state-licensed pharmacies. Prescription medication only available if prescribed after an online consultation with a healthcare provider. Physicians may prescribe compounded medications as needed to meet patient requirements or drug shortages. The FDA does not review or approve any compounded medications for safety or effectiveness. Results may vary. Plans are offered as a subscription service which you can cancel at any time. Actual product packaging may appear differently than shown.
Hit your health goals safely & affordably in 3 simple steps
Submit your application and meet with a doctor
Step 3
Complete a quick form and meet with a licensed medical provider 100% online. They’ll determine if a personalized treatment plan is right for you.

Get your medication delivered at home
Step 3
If eligible, your custom prescription will be shipped directly to your door, fast and free.

Lose weight with 24/7 support and ongoing care
Step 3
We’ll be with you every step of the way, with regular check-ins and on-demand medical support to keep you on track.


"I started at 222 pounds in April and now here we are in August and I am down over 40 pounds... I can't remember ever being this low. It's motivating me to do more things, like go to the gym and eat healthy. I swim a little bit more with the kids. It just gives me confidence. Which has been amazing. I'm so lucky to have found Eden.”





"I had my son in 2021 and struggled to lose weight... I was tired of having low self confidence and feeling like garbage all of the time, so I decided I wanted to try Eden. I've lost 45lbs so far and couldn't be happier! This medication has been truly life changing for me!”
Losing weight is an emotional, physical, and personal challenge that goes beyond just diet and exercise
For many, it's tied to medical conditions that make traditional methods less effective. Prescription medication can help address these underlying issues. Your journey to better health is unique, and Eden is here to support you every step of the way to make real progress toward your health goals.”



Begin your medically-led journey today
- 1-on-1 guidance from US-based health experts
- No-cost consultations for continuous treatment optimization
- Fast & reliable deliveries with 24/7 support available
Every Eden plan is built around you. You’ll receive a personalized treatment plan created by a licensed medical provider after a full review of your health profile. If medication is right for you, we’ll craft a custom dosing schedule and ship your treatment straight to your door, through a certified, state-licensed pharmacy. You’ll also get unlimited 24/7 messaging with your dedicated care team - so you’re supported every step of the way.
There are several FDA-approved prescription medications that may be considered by licensed providers for individuals with obesity, a high BMI, or weight-related health conditions. These may include medications with active ingredients such as semaglutide, tirzepatide, liraglutide, setmelanotide, phentermine, orlistat, and naltrexone-bupropion. In certain cases, other medications, including some antidepressants, may be prescribed off-label. All treatment decisions are made by licensed providers based on clinical evaluation and individual health needs.
Your Eden-licensed medical provider will evaluate your health profile and determine whether a prescription medication may be an appropriate part of your overall treatment plan. Some programs offer access to branded GLP-1 medications that are injectable, as well as personalized oral medication kits that may include ingredients like metformin, bupropion, topiramate, vitamin B12, or naltrexone. These oral medications are typically taken once daily and may work in different ways to support weight management.
You can easily cancel your treatment plan from within your online patient portal. There are no cancellation fees or long-term contracts. Please note that canceling your treatment plan in the portal will not cancel any active orders that have already been sent to the pharmacy. If your order is in a pending status, please contact us directly at [email protected] for assistance.
GLP-1 medications may be preferred over other weight loss drugs due to their non-stimulant nature, reducing the risk of side effects related to stimulant medications. Unlike stimulants such as phentermine, GLP-1s may aid in weight loss while also improving blood sugar control. The suitability of medications varies depending on your health profile. Start your free telehealth consultation to discuss available medication options with a licensed provider.
Eden’s network of compounding pharmacies customize your medications to meet your particular health profile and personal preferences. The compounded medications come in various forms, from injectables to liquids. This custom approach also allows our network of doctors to adjust dosages as needed and cater to your specific needs. All medications that Eden provides access to are prepared in compounding pharmacies licensed by the State Board of Pharmacy or in FDA-licensed 503(a) outsourcing facilities. We work with pharmacies licensed in all 50 states that hold special accreditations and go through extensive vetting processes.
Yes! Your licensed medical provider will review your health history and weight loss goals to create a plan that’s tailored to your needs. They can adjust your medication or dosage based on your progress to ensure it continues to support your overall health. Your care team is available 24/7 to answer questions, offer guidance, and help you stay on track safely throughout your journey.
Here at Eden, we offer wide-spanning weight loss solutions including multiple forms of GLP-1. We always recommend connecting with one of our experts to find the medication that suits your needs.
Every person is different. That’s why Eden connects you with board-certified doctors who will review your full health history and determine if a GLP-1 medication is appropriate for you. Only a licensed healthcare provider can determine whether a medication is safe based on your individual medical profile.
Eden offers access to GLP-1-based solutions through a subscription service that you can cancel anytime. Our focus is on helping patients build sustainable habits and achieve lasting results by connecting them with qualified healthcare providers. Maintaining consistency in your treatment plan is key, but the choice remains flexible to suit your needs.
GLP-1 Treatments
- No hidden fees
- Personalized plans
- On-demand medical support
- Free expedited shipping
Only available if prescribed after an online consultation with a healthcare provider. Benefits outlined are based on third-party studies. Plans are offered as a subscription service which can be canceled at any time. Actual product packaging may appear differently than shown. Physicians may prescribe compounded medications as needed to meet patient requirements. The FDA does not review or approve any compounded medications for safety or effectiveness. The statements on this page have not been evaluated by the FDA. Results may vary. If you notice any side effects while using this treatment, contact your healthcare provider immediately.